EASYGEN is a European research project developing a modular platform for hospital-based CAR-T manufacturing. Led by Fresenius and Fraunhofer IZI, it brings together 18 partners from academia and industry.
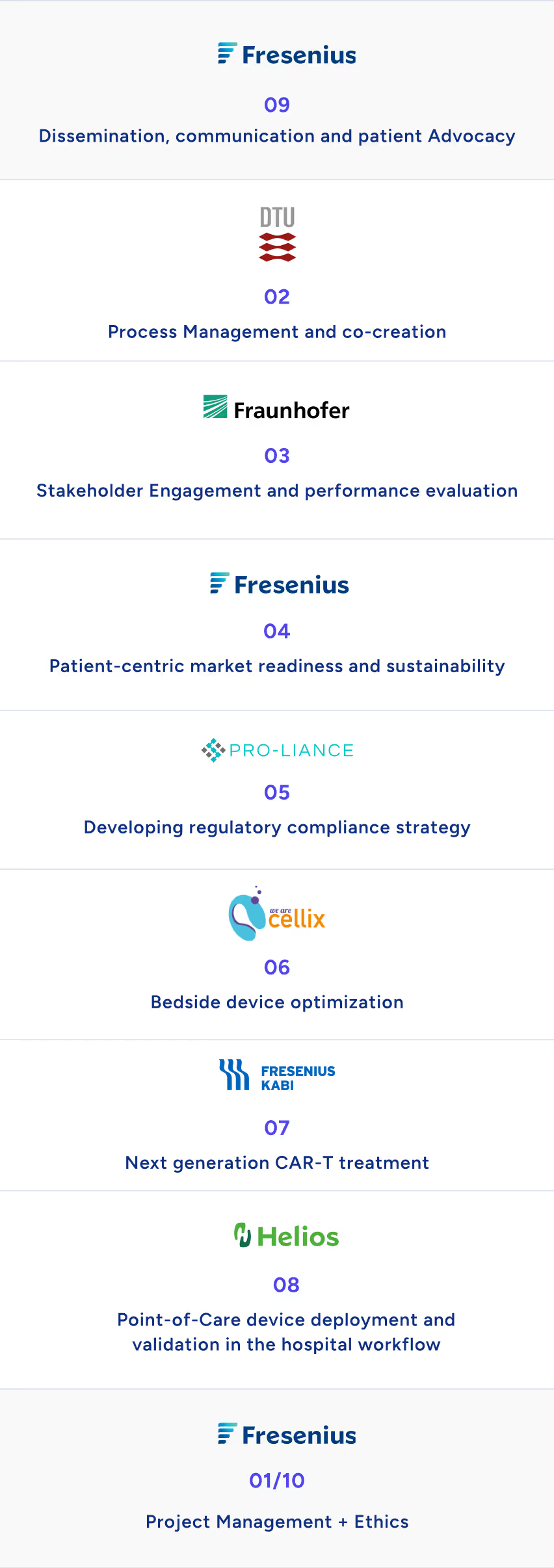
See our work packages
18 partnersEurope-wide collaboration across research, healthcare, industry, and policy.
5 yearsProject duration, enabling full development, integration, and evaluation.
IHI & EU fundedA public-private partnership supported by the EU and industry.





01
Project management
WP1
Project management
Facilitating seamless governance, oversight, and ethical integrity.
This work package establishes the project’s governance framework: the coordinator (FSE) oversees day-to-day administration, budget, and legal compliance, and scientific stewardship - organizing meetings, managing contracts, and ensuring that deliverables, milestones, and reports are delivered on time. It also sets up internal communication channels to keep all partners aligned, while implementing a proactive risk-management cycle (identification, analysis, mitigation, control) to address emerging threats. A data management plan is created and maintained to ensure consistency, accessibility, and integrity of project data across all work packages (WP1 - WP10). Finally, the setup of a scientific advisory board and ethics comittee ensures continuous scientific guidance and guarantees to uphold ethical standards throughout the project lifecycle.
02
Process management and co-creation
WP2
Process management and co-creation
Optimizing care pathways through data-driven simulation and stakeholder collaboration.
Current and future CAR-T care processes are mapped, analyzed and enhanced under real-world constraints. Through expert interviews, observational studies, and KPI definition, partners will build detailed process diagrams and quantitative baselines to inform discrete-event simulation models. These models will be calibrated, used for “what-if” analyses to develop resource concepts (layout, staffing, logistics) and contribute to the performance evaluation in WP3. In parallel, qualitative co-creation workshops with clinicians ensure that user insights drive the design of optimized workflows, SOPs, and training materials.
03
Stakeholder engagement and performance evaluation
WP3
Stakeholder engagement and performance evaluation
Defining needs, acceptance, and economic value for successful technology uptake.
A PESTEL analysis and expert interviews map out all external influences and key actors to pinpoint the political, economic, social and regulatory factors that will shape technology adoption. It then translates stakeholder insights into prioritized technical and usability requirements via targeted questionnaires, ensuring the device meets real-world needs. Building on this, acceptance studies systematically gather and analyze stakeholders feedback to establish clear criteria for end-user buy-in, and a comprehensive cost-effectiveness model compares point-of-care versus conventional CAR-T therapies (including QALYs, long-term follow-ups, Monte Carlo sensitivity analysis, and workflow-driven cost inputs) to guide strategic decision-making.
04
Patient-centric market readiness and sustainability
WP4
Patient-centric market readiness and sustainability
Paving the way for broad, lasting adoption of Point-of-Care CAR-T therapy
WP4 defines and validates patient eligibility criteria to identify new cohorts who stand to benefit from the modular CAR-T device, leveraging clinical expertise and data analytics to model anticipated outcomes. A comprehensive market and competitor analysis maps current trends, barriers to entry, and growth projections, informing optimal positioning within healthcare systems. Finally, insights from WP2 are used for exploitation and sustainability strategies that outline clear commercialization pathways, partner engagement, and scalable roll-out plans to ensure long-term integration and impact of the innovation.
05
Developing regulatory compliance strategy
WP5
Developing regulatory compliance strategy
Charting a clear path to CE-marking and clinical approval
A robust regulatory roadmap is crafted for both the CGT proof-of-concept platform and the modular CAR-T procedure. Early engagement with Notified Bodies and competent authorities ensures that device classification, pre-clinical testing, and evolving regulations are addressed in real time. A detailed European regulatory landscape analysis across EU countries (starting with Germany, Spain) informs consultation with TÜV, PEI, EMA, and other agencies, while comprehensive GMP documentation - including validation plans, risk assessments, and supplier qualification - lays the groundwork for compliant manufacturing in WP6.
06
Point-of-care device optimization
WP6
Point-of-care device optimization
Enhancing the point-of-care platform for reliable CAR-T manufacturing and data integration
Refining and validating the consumable cassette and core instrument to meet GMP specifications for on-site CAR-T production is performed in this working package: partners will iterate on the Point-of-Care (PoC) - cell and gene therapy cassette design (affinity capture, washing, concentration) and verify cell-isolation performance (purity, viability, yield) across multiple donor samples. Standardized quality-control cartridges and protocols will be developed to automate cell counts, viability checks, and contamination assays, by stakeholder feedback from WP8. Finally, a Digital Twin will integrate process and quality control data into hospital EHR/LIMS systems, enabling real-time interoperability and streamlined clinical workflows.
07
Next-generation CAR-T treatment
WP7
Next-generation CAR-T treatment
Advancing rapid, high-fidelity manufacturing and analytics for point-of-care therapies
Same-day CAR-T production is established and refined through the comparison of novel vector and gene-editing strategies with classical methods. Both processes are then standardized to ensure consistent, clinical-scale use. It develops cutting-edge analytical assays - leveraging CRISPR off-target profiling, 3D ex vivo efficacy screens, and cytokine-release monitoring - to qualify edited cells for safety and potency. Finally, it conducts paired comparisons of the new rapid workflow versus traditional ex vivo culture to demonstrate equivalence or superiority in product quality.
08
Point-of-care device deployment and validation in the hospital
WP8
Point-of-care device deployment and validation in the hospital
Ensuring real-world readiness through mock runs, user feedback, and seamless data flow
Simulated onboarding workflows in hospital settings serve to pilot the point-of-care CAR-T platform, while also generating SOPs, training materials, and quality-management documents to assess personnel, infrastructure, and documentation requirements. Guided by insights from WP2 and WP3, this WP then conducts dry runs across partner sites, gathering usability feedback to iteratively refine device deployment and clinical processes while preparing for a clinical trial. Finally, interoperability tests validate data interfaces between the device, digital twin, and hospital IT systems, and a clinical study protocol is drafted alongside a comprehensive final report outlining logistics, user insights, and regulatory considerations.
09
Dissemination, communication, and patient advocacy
WP9
Dissemination, communication, and patient advocacy
Amplifying impact through transparent outreach and stakeholder collaboration
To share EASYGEN’s findings with scientific, clinical, and public audiences the team develops a dynamic dissemination and communication strategy – featuring a unified brand identity, website, social media presence and toolkit for presentations and press releases. It conducts a literature review on CAR-T patient quality of life to inform targeted messaging and convenes roundtable workshops with patient advocacy groups to co-create educational resources and a white paper on lived-experience insights. Ongoing stakeholder networking ensures that healthcare providers and patients are actively engaged throughout the project’s lifespan.
10
Ethics requirements
WP10
Ethics requirements
Embedding ethical integrity across all project activities
To ensure full compliance withapplicable laws, regulations, and best practices, this working package defines an ethical framework and oversight structure guiding all research and device development activities. An ethics committee will be convened to review protocols, informed-consent materials, data-protection measures (e.g., GDPR compliance), and patient-safety procedures, providing guidance and approval at key milestones. Continuous monitoring and reporting mechanisms will be implemented to address any emerging ethical issues throughout the project lifecycle.
01
Project management
WP1
Project management
Facilitating seamless governance, oversight, and ethical integrity.
This work package establishes the project’s governance framework: the coordinator (FSE) oversees day-to-day administration, budget, and legal compliance, and scientific stewardship - organizing meetings, managing contracts, and ensuring that deliverables, milestones, and reports are delivered on time. It also sets up internal communication channels to keep all partners aligned, while implementing a proactive risk-management cycle (identification, analysis, mitigation, control) to address emerging threats. A data management plan is created and maintained to ensure consistency, accessibility, and integrity of project data across all work packages (WP1 - WP10). Finally, the setup of a scientific advisory board and ethics comittee ensures continuous scientific guidance and guarantees to uphold ethical standards throughout the project lifecycle.
02
Process management and co-creation
WP2
Process management and co-creation
Optimizing care pathways through data-driven simulation and stakeholder collaboration.
Current and future CAR-T care processes are mapped, analyzed and enhanced under real-world constraints. Through expert interviews, observational studies, and KPI definition, partners will build detailed process diagrams and quantitative baselines to inform discrete-event simulation models. These models will be calibrated, used for “what-if” analyses to develop resource concepts (layout, staffing, logistics) and contribute to the performance evaluation in WP3. In parallel, qualitative co-creation workshops with clinicians ensure that user insights drive the design of optimized workflows, SOPs, and training materials.
03
Stakeholder engagement and performance evaluation
WP3
Stakeholder engagement and performance evaluation
Defining needs, acceptance, and economic value for successful technology uptake.
A PESTEL analysis and expert interviews map out all external influences and key actors to pinpoint the political, economic, social and regulatory factors that will shape technology adoption. It then translates stakeholder insights into prioritized technical and usability requirements via targeted questionnaires, ensuring the device meets real-world needs. Building on this, acceptance studies systematically gather and analyze stakeholders feedback to establish clear criteria for end-user buy-in, and a comprehensive cost-effectiveness model compares point-of-care versus conventional CAR-T therapies (including QALYs, long-term follow-ups, Monte Carlo sensitivity analysis, and workflow-driven cost inputs) to guide strategic decision-making.
04
Patient-centric market readiness and sustainability
WP4
Patient-centric market readiness and sustainability
Paving the way for broad, lasting adoption of Point-of-Care CAR-T therapy
WP4 defines and validates patient eligibility criteria to identify new cohorts who stand to benefit from the modular CAR-T device, leveraging clinical expertise and data analytics to model anticipated outcomes. A comprehensive market and competitor analysis maps current trends, barriers to entry, and growth projections, informing optimal positioning within healthcare systems. Finally, insights from WP2 are used for exploitation and sustainability strategies that outline clear commercialization pathways, partner engagement, and scalable roll-out plans to ensure long-term integration and impact of the innovation.
05
Developing regulatory compliance strategy
WP5
Developing regulatory compliance strategy
Charting a clear path to CE-marking and clinical approval
A robust regulatory roadmap is crafted for both the CGT proof-of-concept platform and the modular CAR-T procedure. Early engagement with Notified Bodies and competent authorities ensures that device classification, pre-clinical testing, and evolving regulations are addressed in real time. A detailed European regulatory landscape analysis across EU countries (starting with Germany, Spain) informs consultation with TÜV, PEI, EMA, and other agencies, while comprehensive GMP documentation - including validation plans, risk assessments, and supplier qualification - lays the groundwork for compliant manufacturing in WP6.
06
Point-of-care device optimization
WP6
Point-of-care device optimization
Enhancing the point-of-care platform for reliable CAR-T manufacturing and data integration
Refining and validating the consumable cassette and core instrument to meet GMP specifications for on-site CAR-T production is performed in this working package: partners will iterate on the Point-of-Care (PoC) - cell and gene therapy cassette design (affinity capture, washing, concentration) and verify cell-isolation performance (purity, viability, yield) across multiple donor samples. Standardized quality-control cartridges and protocols will be developed to automate cell counts, viability checks, and contamination assays, by stakeholder feedback from WP8. Finally, a Digital Twin will integrate process and quality control data into hospital EHR/LIMS systems, enabling real-time interoperability and streamlined clinical workflows.
07
Next-generation CAR-T treatment
WP7
Next-generation CAR-T treatment
Advancing rapid, high-fidelity manufacturing and analytics for point-of-care therapies
Same-day CAR-T production is established and refined through the comparison of novel vector and gene-editing strategies with classical methods. Both processes are then standardized to ensure consistent, clinical-scale use. It develops cutting-edge analytical assays - leveraging CRISPR off-target profiling, 3D ex vivo efficacy screens, and cytokine-release monitoring - to qualify edited cells for safety and potency. Finally, it conducts paired comparisons of the new rapid workflow versus traditional ex vivo culture to demonstrate equivalence or superiority in product quality.
08
Point-of-care device deployment and validation in the hospital
WP8
Point-of-care device deployment and validation in the hospital
Ensuring real-world readiness through mock runs, user feedback, and seamless data flow
Simulated onboarding workflows in hospital settings serve to pilot the point-of-care CAR-T platform, while also generating SOPs, training materials, and quality-management documents to assess personnel, infrastructure, and documentation requirements. Guided by insights from WP2 and WP3, this WP then conducts dry runs across partner sites, gathering usability feedback to iteratively refine device deployment and clinical processes while preparing for a clinical trial. Finally, interoperability tests validate data interfaces between the device, digital twin, and hospital IT systems, and a clinical study protocol is drafted alongside a comprehensive final report outlining logistics, user insights, and regulatory considerations.
09
Dissemination, communication, and patient advocacy
WP9
Dissemination, communication, and patient advocacy
Amplifying impact through transparent outreach and stakeholder collaboration
To share EASYGEN’s findings with scientific, clinical, and public audiences the team develops a dynamic dissemination and communication strategy – featuring a unified brand identity, website, social media presence and toolkit for presentations and press releases. It conducts a literature review on CAR-T patient quality of life to inform targeted messaging and convenes roundtable workshops with patient advocacy groups to co-create educational resources and a white paper on lived-experience insights. Ongoing stakeholder networking ensures that healthcare providers and patients are actively engaged throughout the project’s lifespan.
10
Ethics requirements
WP10
Ethics requirements
Embedding ethical integrity across all project activities
To ensure full compliance withapplicable laws, regulations, and best practices, this working package defines an ethical framework and oversight structure guiding all research and device development activities. An ethics committee will be convened to review protocols, informed-consent materials, data-protection measures (e.g., GDPR compliance), and patient-safety procedures, providing guidance and approval at key milestones. Continuous monitoring and reporting mechanisms will be implemented to address any emerging ethical issues throughout the project lifecycle.
EASYGEN brings together cross-disciplinary expertise to turn a fragile, complex CAR-T supply chain into a scalable hospital procedure.
More about our goalsCell-engineering pioneers
provide the know-how for robust, high-yield protocols
Automation Experts
Translate biology into aseptic machines and smart sensors.
IT & Software Teams
Co-design e-batch records that integrate with hospital systems.
Regulatory & QA Specialists
Ensure decentralised production meets EU-GMP and ATMP standards.
Health Economists & Payers
Model affordability and system-wide sustainability.
Clinicians & Nurses
Refine patient-centred workflows for everyday hospital use.

In the front row, from left to right: Dr. Sonja Steppan (Easygen Principal Investigator, Fresenius SE), Prof. Dr. Michael Hudecek (Fraunhofer IZI), Theresa Kagerbauer (TQ Therapeutics), Dr. Agnes Vosen (HZDR), Christopher Wegener (Fresenius Kabi), Vaclovas Radvilas (EBMT), Dr. Julia Schüler (Charles River), Dr. Julia Busch-Casler (HZDR), Nicole Spanier-Baro (Fraunhofer IESE), Vivienne Williams (Cellix Limited), Prof. Dr. Bertram Glaß (Helios), Prof. Dr. Ulrike Köhl (Fraunhofer IZI), Rebecca Scheiwe (Fresenius SE). In the back row, from left to right: Prof. Dr. Ralf Kuhlen (Fresenius SE), Prof. Dr. Jens O. Brunner (DTU), Dominik Narres (Fresenius SE), Thomas Brzoska (Pro-Liance Global Solutions), Dr. David Krones (Fraunhofer IZI), Dr. Sabine Bertsch (Pro-Liance Global Solutions), Dr. Ralf Hoffmann (Philips), Christin Zündorf (TQ Therapeutics), Dr. Anna Dünkel (Fraunhofer IZI).






